Will Big Data Finally Demystify the Brain and Alzheimer’s?
Digital imaging could revolutionize how we detect and treat dementia and neurologic diseases.
It all started 30 years ago with a conversation between scientists over a beer.
Arthur Toga listened, intrigued, as one of his colleagues described a then-new project that used a specialized computer system to link together early satellite imagery of the Earth. A neuroscientist, Toga wondered if that computer system could do the same with pictures from the human body—such as images that capture rates of metabolism in the brain.
What would we be able to see, Toga pondered, if we could use computer-enabled imaging to view how the brain works?
Toga has devoted his career to answering that question. As director of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute, he has built one of the largest data collections of human brain images in the world. By combining the power of computer science with the study of brain science, his team at the institute—which comprises researchers across disciplines like neuroscience, physics, engineering and math—can map brain image data in an unprecedented way. The scientists combine images showing brain structure and function with other measures like cognition, behavior and genetics.
Of course, medical imaging isn’t new. Neurologists already use it to assess damage and recommend treatment for people who have had a stroke, for example. But Toga wants to make the most of these scans through the power of volume.
All images taken in the National Institutes of Health-funded stroke clinical trials, the Alzheimer’s Disease Neuroimaging Initiative and the Parkinson’s Progression Markers Initiative—thousands of scans—are centralized in Toga’s lab at the USC Stevens Neuroimaging and Informatics Institute. Patterns found across these scans could lead to ways to diagnose and treat these conditions more effectively.
And that’s just one of the many projects underway at the USC Stevens Neuroimaging and Informatics Institute, where more than a hundred investigators led by Toga and Paul Thompson, associate director, pursue scientific mysteries every day. Based at the Keck School of Medicine of USC, their quest to digitize and map the brain could find answers to neurodegenerative diseases, among the most intractable problems in human health—and the most costly.
Seeing the Brain
Worldwide, more than 47 million people suffer from Alzheimer’s disease and other forms of dementia, and that number is predicted to triple by 2050. Yet society is unprepared for it. Part of the problem with prevention and treatment of these diseases is that doctors don’t know the exact factors that lead to them. Likewise, when illness arises, it’s difficult to pinpoint the changes underway until late in the process.
What’s especially heartbreaking about brain diseases is that they can alter people’s core traits, says USC Provost Michael Quick, a professor of biological sciences at USC Dornsife College of Letters, Arts and Sciences. “If you have kidney disease, or diabetes, we don’t tend to think of you as a changed person because of it. But when talking about a disease of the brain, it’s something that is affecting, potentially, who that person is.”
That’s because everything from our memories to our personality is wrapped up within the networks that make the brain tick. And there are ever-growing ways to analyze these networks through imaging.
“We can measure the brain’s grey matter and white matter, but we could also map the anatomy of connections—the wiring diagram,” Toga says.
Imaging devices can see much more than the structure of the brain, he explains. They can see what’s happening in the brain at any moment in time, like where there’s more blood flow or electrical activity. The goal, he says, is to integrate these layers of data and create a rich map of the brain that’s constantly changing.
Combining structure and function into one map shows what the brain is doing when it’s functioning well, and what goes awry when there’s a problem. Today the only way scientists can uncover the subtle nuances that distinguish a healthy brain from a brain heading toward disease is by mining through many thousands of brain images—research on a massive scale made possible at the USC Stevens Neuroimaging and Informatics Institute.
These brain imaging studies also can help researchers understand how the normal, healthy brain varies from person to person. Some people stay mentally sharp into their late years, and understanding the structure and function of their brains could help others live longer with a better quality of life. Then there’s the issue of how the brain develops.
Throughout a person’s life, the brain changes—a baby’s brain isn’t like the brain of an older person, healthy or unhealthy—and scientists can learn a lot about the brain by mapping it as it grows.
This kind of mapping was once inconceivable. The computational power needed to crunch data and store huge images became available only recently. The USC Stevens Neuroimaging and Informatics Institute stores four petabytes of data—the equivalent of about 53 years’ worth of high-definition video—and it will keep growing.
Yet researchers are still struggling to process and share the immense amount of imaging data being gathered around the world. In 2014, Toga won a $12 million grant from the National Institutes of Health to develop systems and strategies at the USC Laboratory of Neuro Imaging, part of the USC Stevens Neuroimaging and Informatics Institute, to help scientists and physicians mine this brain-related big data.
Even though great strides have been made, scientists also know they could do more with better technology. Today’s equipment lets them see the brain as if they were on an airplane at 37,000 feet: It’s an expansive view, but not detailed. “Imaging is a technology that is continuing to evolve in resolution and sensitivity,” Toga says. “We can’t see individual cells at the cellular or molecular level using MRI.”
The next step may involve creating new detection technologies and tracers—molecular homing beacons—to witness the brain at this small scale, Toga explains. Ultimately, the ability to see cellular or even molecular underpinnings of brain function may provide the targets that scientists need to create therapies against neurological disease or psychiatric disorders.
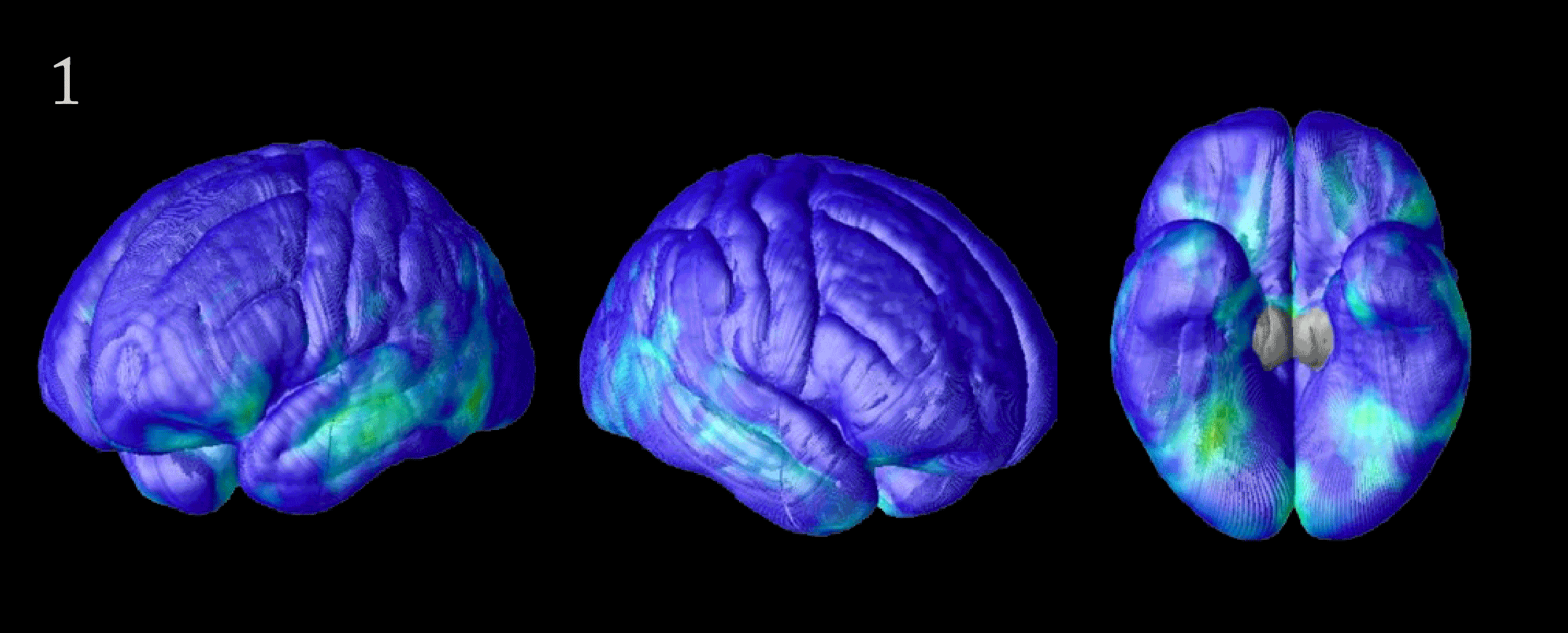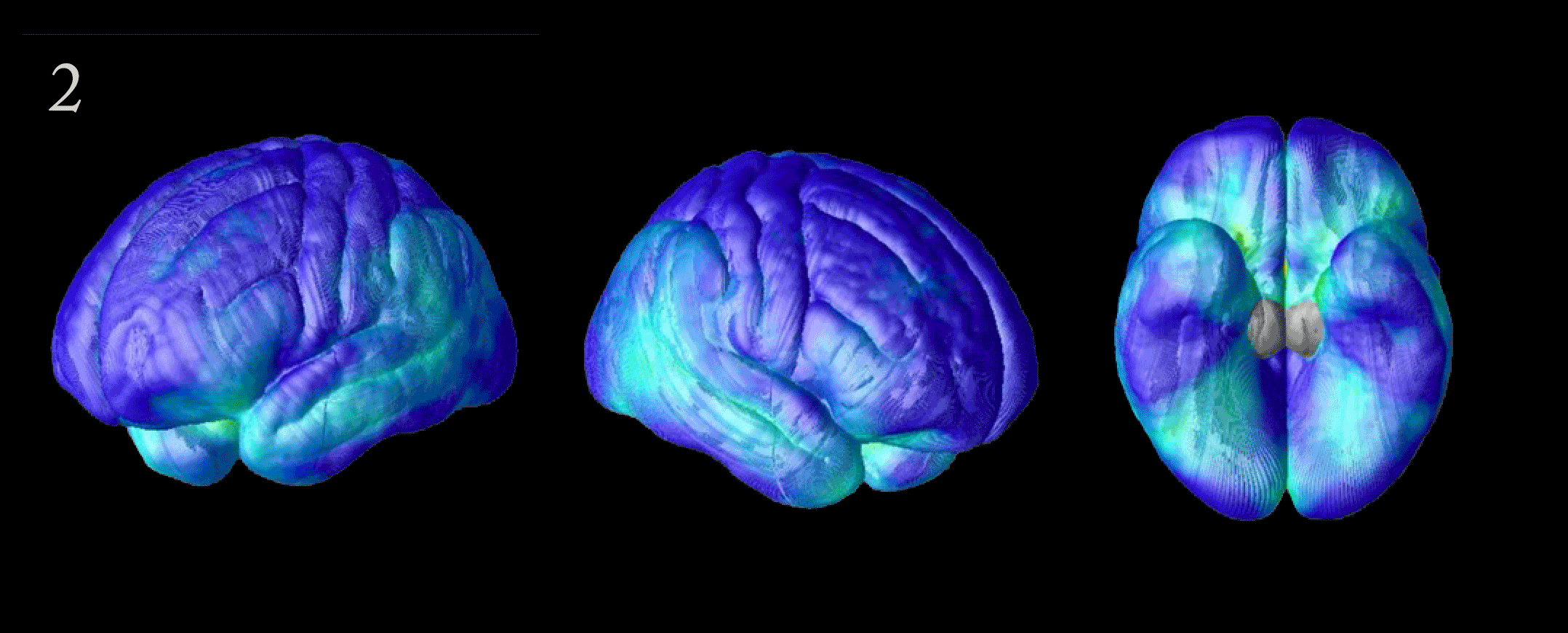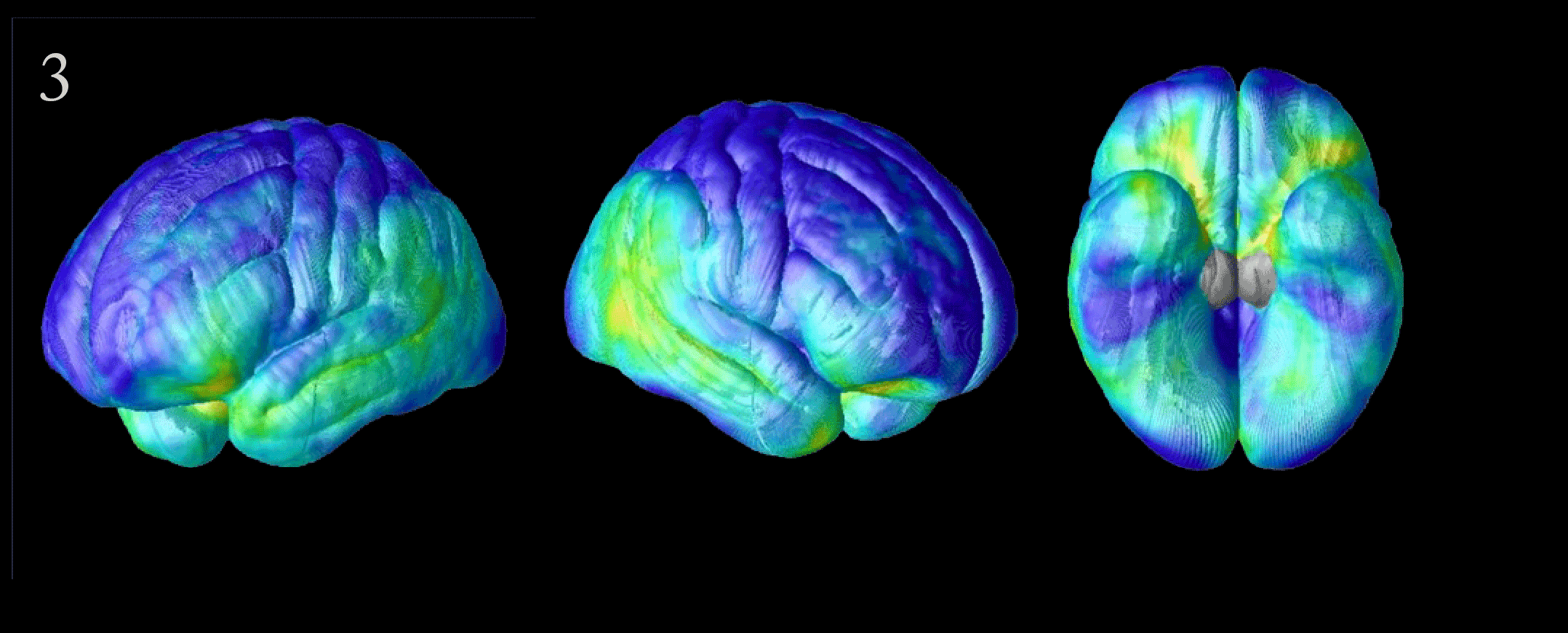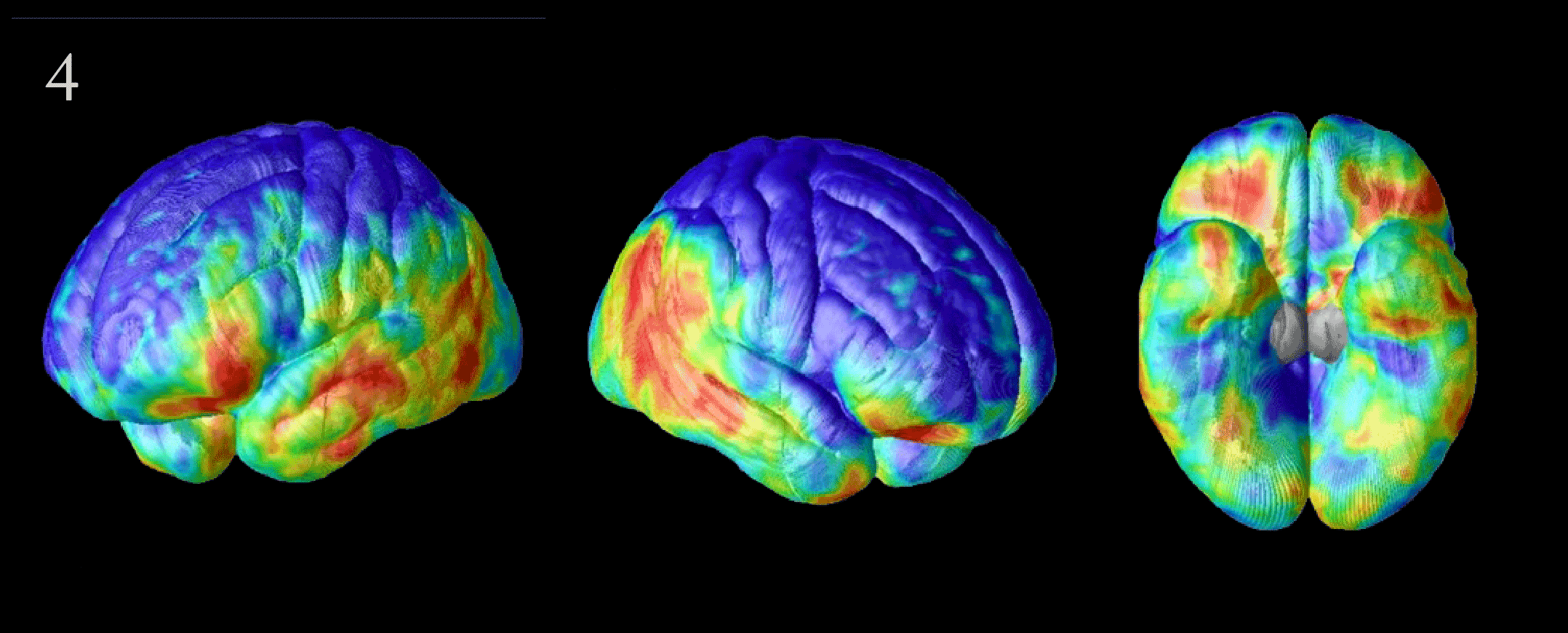
Flood of Data
Some six years ago, Toga’s fellow USC neuroscientist Paul Thompson was chatting with esteemed Australian geneticist Nick Martin when Martin made an offhand comment: While geneticists share data all the time, people working in brain science often don’t.
That got Thompson thinking. Sharing could potentially be a tool for solving the brain’s mysteries the same way it fast-tracked the decoding of the human genome. “If you had enough genetic data and enough scan data, you could crack the brain’s genetic code,” Thompson mused.
The cipher-breaking reference during that 2009 conversation inspired the name ENIGMA, which is now one of the world’s largest brain imaging studies. Led by Thompson, the Enhancing Neuro Imaging Genetics through Meta Analysis project won an $11 million grant from the National Institutes of Health last fall to develop new computational algorithms to find biomarkers of mental illnesses and brain diseases.
Like the famous enciphering machine from World War II, ENIGMA revolves around code. But in this case, it relies on sophisticated technology—and new ways to analyze data—to reach a deeper understanding of the brain.
The project documents how a dozen diseases, including Alzheimer’s, schizophrenia, depression and bipolar disorder, alter the brain. In January, after combing through brain scans and DNA of 30,000 people around the globe, the ENIGMA researchers found eight genetic variants that determine the size of different regions of the brain. These genetic clues may tip off scientists about who will benefit from current drugs and how to develop better medicines. More findings are on the way.
Since the ENIGMA project brings together experts from 35 countries and thousands of patients’ brain scans every day, Thompson likens it to crowdsourcing a problem—using everyone’s abilities to maximize the output. Multiple sclerosis is a good example: By pooling imaging, scientists can look for patterns and places where the disease is more common. That could lead to theories about why it occurs. “The ENIGMA project is work that’s truly impactful. It’s collecting data from across the world to address issues that have global consequences,” says Quick, USC’s provost.
Sometimes trawling through big data yields surprises, like when ENIGMA researchers studied patients who were taking drugs to manage bipolar illness and schizophrenia. The scientists wanted to find out which medications worked best to restore brain function or stop the loss of brain tissue. Along the way, they found that there’s a surprisingly robust pattern of physical brain changes in people with schizophrenia, including alterations to areas that control memory. Thompson notes that without such a vast source of data, it would be hard to know if those changes were actually due to the disease or to something else.
The researchers also never expected to find that the hippocampus—the brain’s memory center—is smaller than average in people who are depressed, and even smaller if they became depressed early in life. The longer people suffer from the illness, the more their brain differs from the typical brain. That finding gets Thompson excited because it suggests solutions. “If there is a way to treat depression early, you could slow the progression of brain tissue deterioration,” he says.
Then there’s Alzheimer’s disease. Using scans, ENIGMA researchers charted what Thompson calls a “forest fire of tissue loss” in brains with Alzheimer’s. The disease first attacks the parts of the brain that guide memory, then the ones that govern emotions, and then the disease skips over sensory-controlling areas of the brain until it finally degrades the frontal areas involved in self-control and inhibition. “As this cascade of events happens, you see this devastating decline in a person’s ability to handle everyday life,” Thompson says. Unfortunately, while imaging can clearly show how the brain degrades in Alzheimer’s, he says, no treatments can yet stop it.
Big data holds promise, though. Databases at the USC Stevens Neuroimaging and Informatics Institute not only include images of the brains of patients with diseases like Alzheimer’s, but also information about each patient’s genetic makeup. That means the scientists can study vast numbers of DNA sequences. In this case, the researchers can search through brain images of people who have genes that predispose them to Alzheimer’s disease. Some of these genes match up to specific physical changes in the brain. As the scientists gather more data, these genes could suggest targets for treatment.
The deluge of data across bioscience could produce a river of innovation, or a flood of information that rushes by, unused, until science can catch up to it. USC Stevens Neuroimaging and Informatics Institute researchers are partnering with researchers across the world to seize these opportunities. After all, millions of people are waiting for answers.
Michael Quick sees parallels in earlier bioscience research. “A hundred years ago, when we started using X-rays, we were throwing away all sorts of data because we didn’t have the capacity to use them,” he says.
“But as time has marched on, we have been able to mine large amounts of the information—and that’s only going to grow.”
