Tiny probe developed at USC detects cancer
USC team’s work could lead to noninvasive detection and treatment of the disease at the level of a single cell
Fabien Pinaud’s big vision for treating cancer homes in on the smallest of targets.
Along with a team of scientists, he created a new hybrid nano-probe that could lead to noninvasive detection and treatment of the disease at the level of a single cell.
Pinaud, assistant professor of biological sciences, chemistry and physics and astronomy at the USC Dornsife College of Letters, Arts and Sciences, developed a method for amplifying a biochemical signal on the surface of cancer cells.
The new technique binds and assembles gold nanoparticles in living cells using two fragments of a fluorescent protein as “molecular glue.” These tiny probes act as amplifiers, enhancing researchers’ ability to detect distinct biomarkers — things like overexpressed or mutated proteins — found in cancer cells.
The boosted signal allows the scientists to distinguish cancer cells from healthy cells through the use of Raman spectroscopy — a specialized laser-imaging technique.
“Our approach takes advantage of the fact that we have two different nanoparticles which, on their own, are not active, but which become active when they assemble on cancer cells,” said Pinaud, principal investigator of the Single Molecule Biophotonics Group and co-author of a related study published in Nature Communications.
Right on target
Using “molecular glue” assemblies to design novel nano-probes is common practice in current biomedical research, but most scientists build these with DNA rather than protein. While promising optical probes are being generated using DNA assemblies in test tubes, DNA is not a practical adhesive in live cancer cells. Proteins are often better.
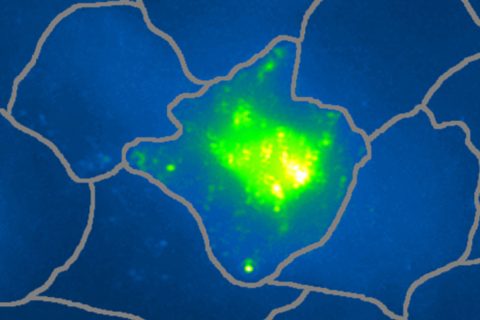
Pinaud and his team start with a fluorescent protein, one that glows when ultraviolet-blue light shines on it. The fluorescent protein is split into two fragments and each piece is attached to a set of gold nanoparticles. Both sets of nanoparticles zero in on cells and bind specifically to biomarkers at the cell surface. As the nanoparticles collide on a cancer cell, the protein fragments naturally reassemble into the whole fluorescent protein.
The restructuring process provides two advantages. First, the activation of a new biochemical signal in the fluorescent protein is massively amplified by the nanoparticles, which allows for detection by Raman imaging.
Second, heat and ultrasounds are produced when the laser hits the nanoparticles, and that can be measured with ultrasound detectors. This dual effect provides confidence that a detected cell is actually cancerous and not a false-positive signal from a healthy cell.
Scientists will next explore the possibility of destroying individual cancer cells, while leaving healthy cells unharmed, by using the laser to heat up the nanoparticles.
“Going from imaging to killing cells is just about turning the knob on the laser that you use,” Pinaud said.
The Single Molecule Biophotonics Group is housed within the Molecular and Computational Biology section at USC Dornsife.
Additional authors on the study include Natalie Tang and Richard Martel from the University of Montréal and Chao Tian, Wei Zhang and Xueding Wang from the University of Michigan.
The work was supported by the National Science Foundation, Division of Material Research, under grant No. 1406812. Tang and Martel were supported by the Natural Sciences and Engineering Research Council of Canada, Canada Research Chair and the Ministère du Québec de l’Économie, Science et Innovation (PSR-SIIRI-810). Tian, Zhang and Wang were supported by National Institutes of Health grants R01CA186769 and R01AR060350.



